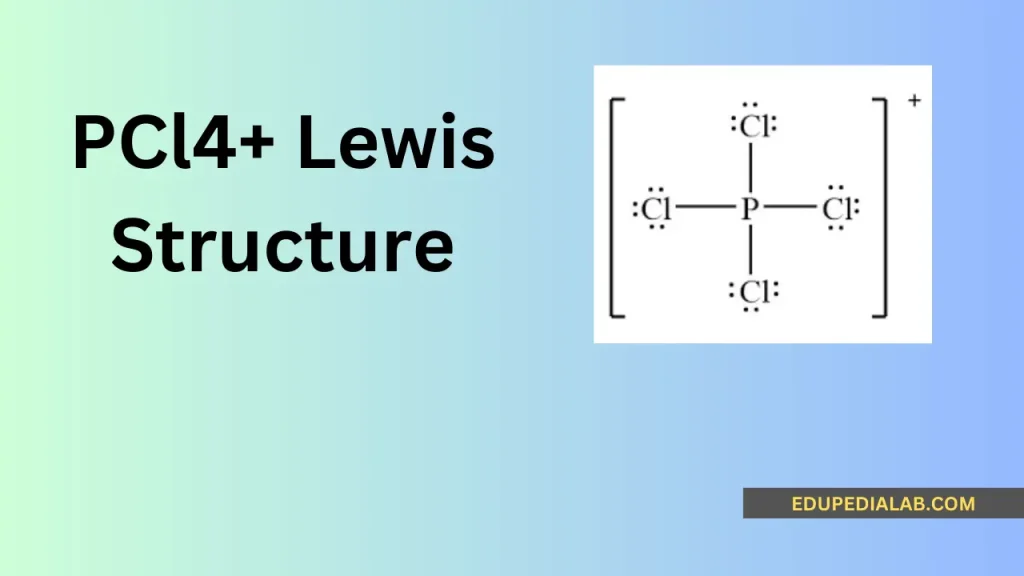
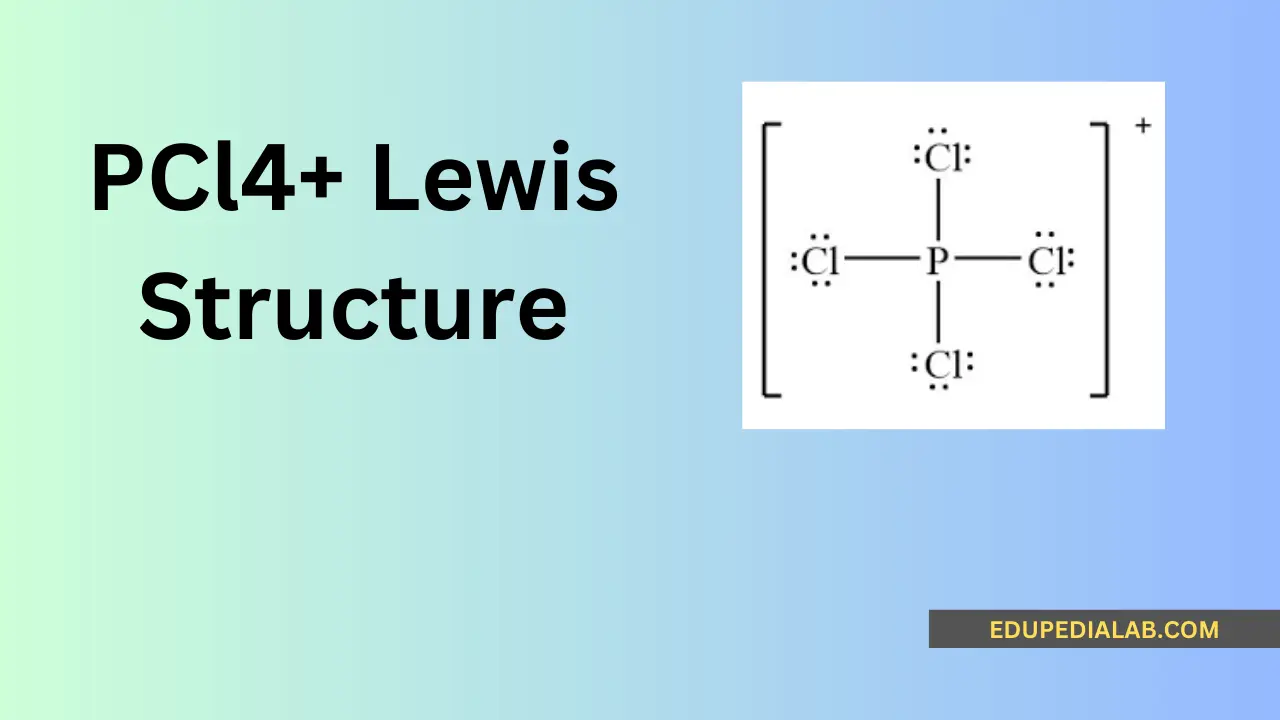
Understanding the Lewis structure of pcl4+ involves analyzing the arrangement of its electrons and atoms to represent the chemical bonding and geometry. The Lewis structure provides essential insights into the compound’s valence electrons, bonding, and overall molecular configuration.
What is a Lewis structure and how is it related to pcl4+?
Explanation of Lewis structure and its significance
The Lewis structure is a diagrammatic representation of a molecule, illustrating the arrangement of its atoms, electrons, and chemical bonds. It helps in understanding the sharing or transfer of valence electrons between atoms and determines the molecular geometry.
How to determine the Lewis structure for pcl4+
Determining the Lewis structure for pcl4+ involves understanding the positions of the constituent atoms and the arrangement of their valence electrons to form stable chemical bonds.
Understanding the concept of valence electrons
Valence electrons are the electrons located in the outermost shell of an atom. The number of valence electrons determines the atom’s ability to form chemical bonds and influences its reactivity.
How to draw the Lewis structure for pcl4+?
Identifying the central atom for pcl4+
The central atom in the pcl4+ molecule is phosphorus, which usually forms the core of the compound’s structure and is surrounded by other atoms through chemical bonding.
Determining the number of valence electrons
Phosphorus contributes 5 valence electrons, and the 4 chlorine atoms contribute 7 valence electrons each, totaling 5 + 4(7) = 33 valence electrons for the pcl4+ molecule.
Placing lone pairs and bonding electrons around the central atom
Allocating the electrons to form bonding and non-bonding pairs around the phosphorous atom provides a comprehensive understanding of the pcl4+ molecular structure and its electron distribution.
What are the key features of pcl4+ Lewis structure?
Explaining the concept of octet rule in Lewis structure
The octet rule states that atoms tend to combine in such a way that each atom has eight electrons in its valence shell, resulting in a stable electronic configuration. The pcl4+ Lewis structure follows this rule, providing stability to the molecule.
Understanding the role of cations in Lewis structures
In the pcl4+ Lewis structure, the phosphorus atom is the cation, having lost one electron to have a positive charge, which influences its bonding and reactivity with other atoms.
Identifying any possible ionic or covalent characteristics in pcl4+ Lewis structure
The phosphorus-chlorine bonding in pcl4+ predominantly exhibits covalent characteristics, as the atoms share electron pairs to form stable chemical bonds, contributing to the overall Lewis structure.
What are the common misconceptions about drawing pcl4+ Lewis structure?
Clarifying misconceptions about the position of lone pairs
There might be misconceptions about the placement of lone pairs around the phosphorus atom in the pcl4+ molecule, as the correct positioning determines the overall molecular geometry and stability.
Addressing the confusion regarding the role of phosphorus in the structure
Understanding the crucial role of phosphorus as the central atom and its impact on the electron distribution and bonding is essential to avoid any misconceptions in drawing the pcl4+ Lewis structure.
Explaining the importance of considering hybridization in determining the structure
Hybridization plays a significant role in determining the pcl4+ Lewis structure, affecting the arrangement of electrons and the nature of chemical bonding within the molecule, addressing potential misconceptions related to its structure.
How does pcl4+ Lewis structure vary from other similar molecules?
Analyzing the differences with other phosphorus-based compounds
The Lewis structure of pcl4+ exhibits distinct differences in its electron arrangement and bonding compared to other phosphorus-based compounds, leading to variations in their molecular geometry and reactivity.
Comparing the Lewis structure of pcl4+ with other compound structures
Comparing the Lewis structure of pcl4+ with that of other compounds allows for a comprehensive understanding of the differences in electron distribution, bonding, and overall stability, highlighting the unique features of pcl4+.
Discussing the implications of the Lewis structure on the chemical properties of pcl4+
The pcl4+ Lewis structure significantly influences its chemical properties, such as its reactivity, polarity, and interactions with other molecules, setting it apart from similar compounds and demonstrating the relevance of its distinctive molecular configuration.
3 thoughts on “ pcl4+ lewis structure”