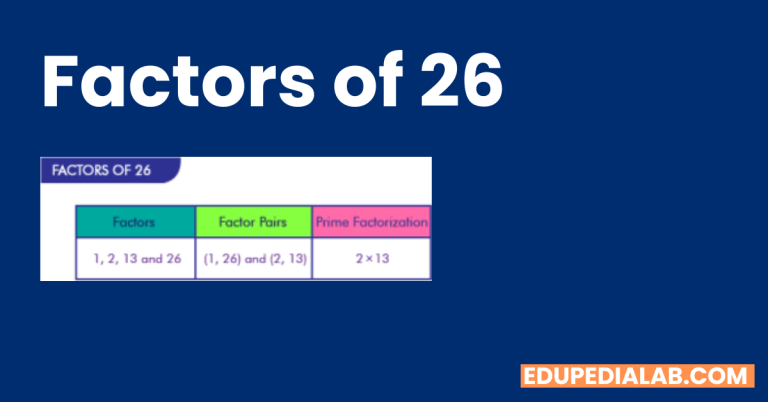
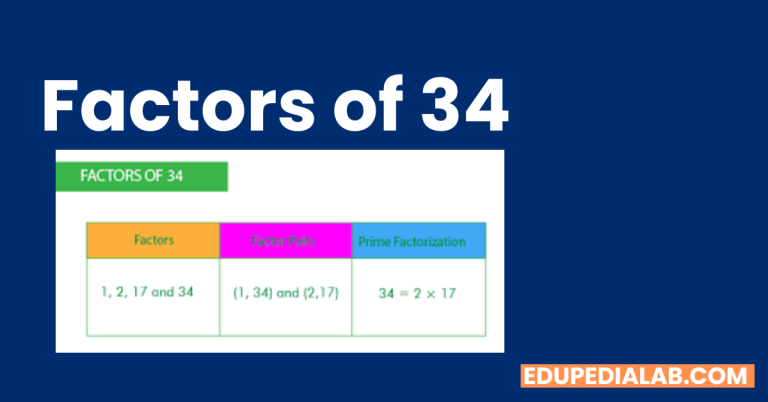
BF3 Polar or Nonpolar
BF3, also known as boron trifluoride, is a molecule with a trigonal planar molecular geometry. It consists of one boron atom and three fluorine atoms. The boron atom is located at the center, while the three fluorine atoms are positioned around it in a flat, triangular arrangement. This molecular structure gives BF3 its trigonal planar…