BF3 Lewis Structure
If you’re studying chemistry or just curious about how molecules are structured, understanding the Lewis structure of BF3 (boron trifluoride) can feel a bit tricky at first. Don’t worry—I’m here to walk you through the process step by step so you can confidently tackle this topic. By the end of this guide, you’ll not only know how to draw the BF3 Lewis structure but also grasp the reasoning behind it.
You can check BF3 Polar Or Non Polar by visiting a comprehensive blog post on our website
What is a Lewis Structure?
Before we jump into BF3 specifically, let’s recap what a Lewis structure is. A Lewis structure is a diagram that represents the valence electrons of atoms within a molecule. It helps us visualize how the atoms bond and how the electrons are distributed—essentially, it’s a handy shortcut for figuring out molecular structure.
Key Facts About BF3
- Molecular formula: BF3
- Atoms involved: Boron (B) and Fluorine (F)
- Total number of valence electrons: 24
- Type of bonds: Covalent bonds
BF3 is an example of a molecule where boron is the central atom. Unlike some other molecules, you’ll notice that BF3 does not follow the “octet rule” strictly, making it an interesting case to study.
How to Draw the BF3 Lewis Structure
Here’s how to break the process down into manageable steps:
Step 1: Count the Total Valence Electrons
First, determine the total number of valence electrons in the molecule.
- Boron (B) is in Group 13 of the periodic table, so it has 3 valence electrons.
- Fluorine (F) is in Group 17, meaning each fluorine atom has 7 valence electrons. Since there are three fluorine atoms, we multiply 7 × 3 = 21 valence electrons from fluorine.
- Add these together:
3 (Boron) + 21 (Fluorine) = 24 valence electrons in total.
Step 2: Place the Least Electronegative Atom in the Center
Boron is less electronegative than fluorine, so boron becomes the central atom. Arrange the three fluorine atoms symmetrically around the boron.
Step 3: Form Single Bonds Between Boron and Fluorine
Next, connect boron to each of the three fluorine atoms with a single bond. Each bond takes 2 electrons. Since we have 3 bonds, we’ve used 6 electrons so far.
Remaining electrons = 24 – 6 = 18 electrons.
Step 4: Distribute the Remaining Electrons Around the Fluorine Atoms
After forming the bonds, distribute the remaining 18 electrons around the fluorine atoms to satisfy their octet rule. Each fluorine atom needs 8 valence electrons (including the 2 electrons it shares in the bond with boron).
- Fluorine #1 gets 6 electrons
- Fluorine #2 gets 6 electrons
- Fluorine #3 gets 6 electrons
Now all the fluorine atoms are “happy,” and the remaining electrons are used up.
Step 5: Check the Central Atom (Boron)
Here’s where BF3 is unique. Boron has only 6 electrons in its outer shell (from the three single bonds). Unlike most atoms, boron is okay with having less than 8 electrons—this is an exception to the octet rule. Scientists call boron an “electron-deficient” element, and that’s perfectly normal in this case!
You don’t need to add more electrons or create double bonds; the structure is complete as it is.
What Does the BF3 Lewis Structure Look Like?
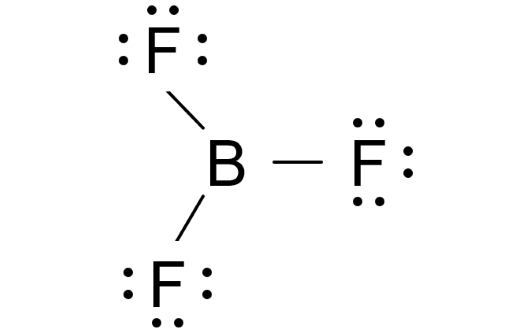
Each fluorine is bonded to boron with a single bond, and their valence shells are filled with electrons. Boron sits in the center with 6 electrons.
Key Points to Remember About BF3
- Doesn’t follow the octet rule: Boron is stable with only 6 electrons.
- Trigonal planar shape: The molecule forms a flat, triangular shape with 120° bond angles.
- Non-polar molecule: While the B-F bonds are polar due to electronegativity differences, the symmetrical shape of BF3 cancels out any dipole moments, making the molecule non-polar overall.
Why is BF3 Important?
BF3 plays an essential role in chemistry, especially in industry and research. It’s commonly used as a Lewis acid (a compound that can accept electrons) in various reactions. Understanding its Lewis structure helps you grasp its reactivity and how it interacts with other molecules.
Final Thoughts
Drawing the BF3 Lewis structure might seem daunting at first, but with practice, it becomes much more intuitive. By following the steps outlined above, you now have a clear and logical approach to understanding this molecule.
If you’re interested in diving deeper into molecular structures, bond angles, or other fascinating topics in chemistry, don’t hesitate to explore more resources. Remember, every expert was once a beginner—keep practicing, and you’ll get there!