C2S Lewis Structure
The C₂S Lewis structure represents the bonding and electron distribution in a molecule composed of two carbon atoms (C) and one sulfur atom (S). This structure is important in understanding how these atoms interact chemically and the overall shape and stability of the molecule. In this guide, we will explore how to draw the C₂S Lewis structure, the key features of this structure, and its significance in chemical reactions.
Do you want to draw the H2S Lewis Structure without doing anything wrong then have a look on our recent blog post
What is the C₂S Lewis Structure?
The C₂S Lewis structure refers to how the atoms in the carbon disulfide (CS₂) molecule are bonded and how their valence electrons are distributed. In this molecule, carbon is the central atom, bonded to two sulfur atoms. Each carbon-sulfur bond in C₂S is a double bond, meaning two pairs of electrons are shared between the carbon and sulfur atoms. Understanding this structure helps explain the molecule’s properties, such as its linear geometry and its behavior in chemical reactions.
Step-by-Step Guide to Drawing the C₂S Lewis Structure
To draw the Lewis structure of C₂S, follow these steps:
1. Count the Total Valence Electrons
Each atom in the molecule contributes electrons from its outermost shell (valence electrons):
- Carbon (C) has 4 valence electrons (since it’s in Group 14 of the periodic table).
- Sulfur (S) has 6 valence electrons (since it’s in Group 16 of the periodic table).
For C₂S, the total number of valence electrons is:
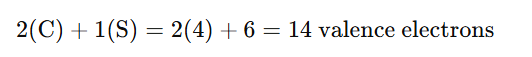
2. Determine the Central Atom
Carbon (C) is less electronegative than sulfur (S), so carbon will be the central atom. This is typical in molecules where carbon forms multiple bonds with other atoms.
3. Connect the Atoms with Bonds
Place the carbon atoms on either side of the sulfur atom and connect them with a single bond. Then, add double bonds between the carbon atoms and sulfur atoms to complete their octets. This gives each sulfur atom two bonds with carbon and each carbon atom four bonds total (two with sulfur and one with the other carbon).
4. Distribute the Electrons
Now that you’ve formed bonds between the atoms, place the remaining electrons to ensure each atom has a full outer shell of electrons:
- Carbon atoms need four electrons each to complete their octet (which they already have by forming double bonds with sulfur).
- Sulfur atoms need two electrons to complete their octet, which they get from the double bonds with carbon.
This results in the following configuration:
- Each carbon atom forms a double bond with one sulfur atom.
- The sulfur atoms each have two lone pairs of electrons.
5. Check for Formal Charges
Ensure the formal charges on each atom are minimized. In this case, the formal charges on carbon and sulfur atoms in C₂S are zero, meaning the structure is stable.
Key Features of the C₂S Lewis Structure
- Bonding: The C₂S molecule has two carbon-sulfur double bonds. This means that each carbon atom shares two pairs of electrons with sulfur, leading to strong covalent bonds.
- Electron Distribution: Each carbon and sulfur atom follows the octet rule, with carbon achieving a stable electron configuration with four bonds and sulfur reaching its octet with two double bonds.
- Molecular Geometry: The C₂S molecule has a linear geometry, with the two carbon atoms and the sulfur atom aligned in a straight line. This results from the sp hybridization of the carbon atoms, which forms 180° bond angles.
- Formal Charges: The formal charges in the C₂S Lewis structure are zero, which indicates that the structure is stable and the electron distribution is ideal.
Significance of the C₂S Lewis Structure
The C₂S Lewis structure is important for understanding the molecule’s chemical properties and how it interacts with other substances. Here are some key aspects:
- Molecular Shape: The linear shape of C₂S makes it nonpolar, as the dipoles in the carbon-sulfur bonds cancel out. This influences how the molecule behaves in chemical reactions and its interactions with other molecules.
- Reactivity: C₂S is reactive due to the presence of strong double bonds between carbon and sulfur. These bonds can participate in nucleophilic reactions, which are common in organic chemistry.
- Industrial Uses: Carbon disulfide (CS₂), which has the same Lewis structure, is used in the production of viscose rayon and cellophane, and as a solvent in various industrial processes.
Common Questions About the C₂S Lewis Structure
1. Why Does Carbon Form Double Bonds with Sulfur in C₂S?
Carbon forms double bonds with sulfur in C₂S to achieve a stable electron configuration and fulfill the octet rule. Carbon has four valence electrons, and by forming two double bonds with sulfur, it can complete its octet and share electrons effectively.
2. What is the Shape of the C₂S Molecule?
The C₂S molecule has a linear geometry, with a bond angle of 180°. This linear shape results from the sp hybridization of the carbon atoms.
3. Is the C₂S Molecule Polar or Nonpolar?
The C₂S molecule is nonpolar due to its linear geometry. The dipoles in the carbon-sulfur bonds cancel each other out, resulting in a molecule that does not have a net dipole moment.
4. How Many Lone Pairs are on the Sulfur Atoms?
Each sulfur atom in the C₂S Lewis structure has two lone pairs of electrons. These lone pairs are important for maintaining the stability of the molecule and ensuring that sulfur completes its octet.
Conclusion
The C₂S Lewis structure is a simple yet crucial representation of the bonding and electron distribution in carbon disulfide. Understanding how to draw this structure and the key features of its bonding provides valuable insight into the molecule’s properties, reactivity, and uses. With its linear geometry, nonpolar nature, and the ability to form double bonds, the C₂S molecule plays a significant role in both organic and industrial chemistry.