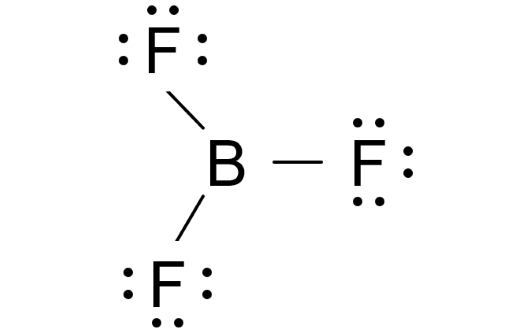
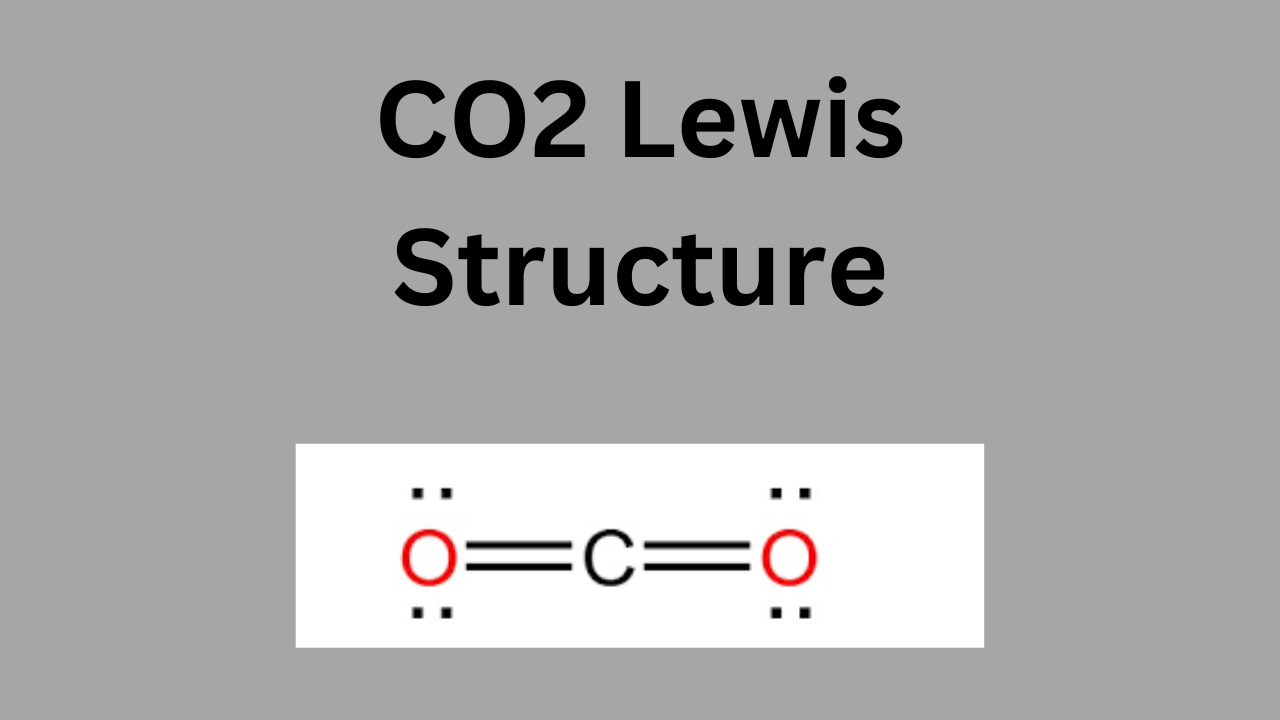
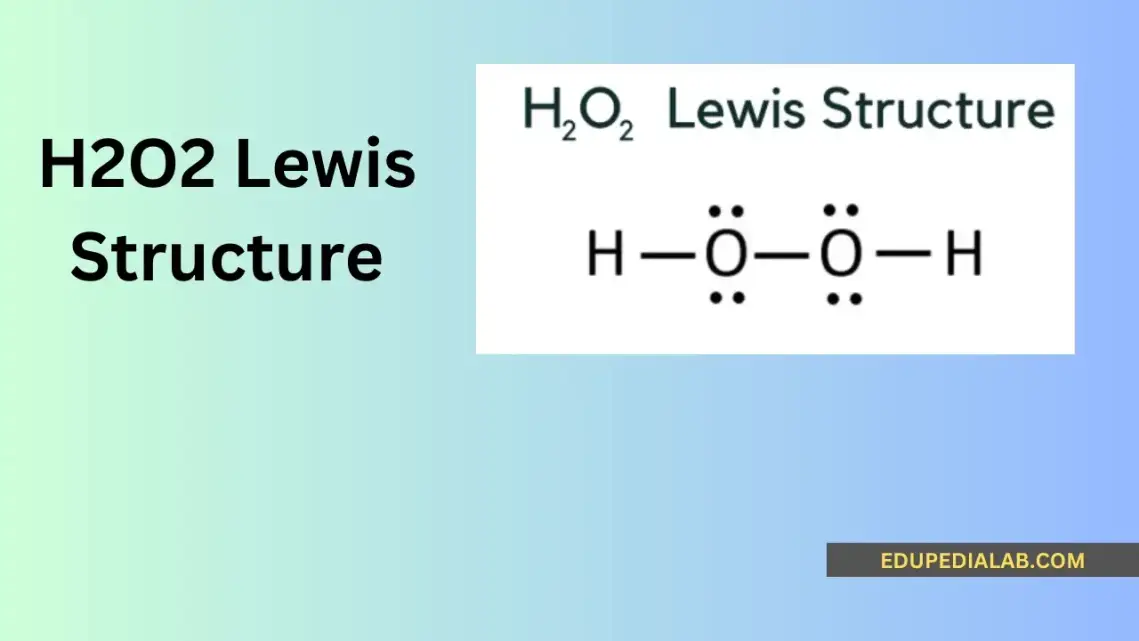
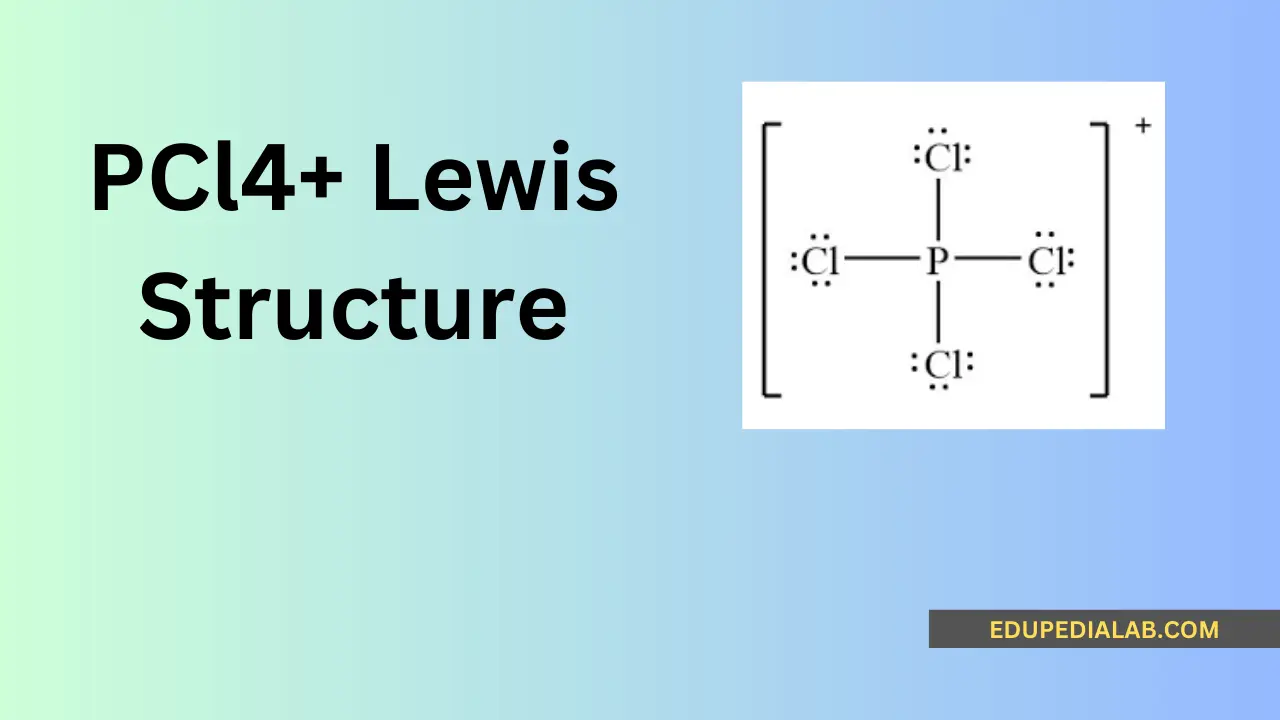
Understanding Polypropylene: Lewis Structure, Properties, and Applications
Polypropylene is one of the most widely used polymers in the world, found in everything from packaging materials to textiles and automotive components. Its versatility, affordability, and durability make it indispensable in modern industries. In this blog post, we will explore polypropylene in detail, starting with its Lewis structure representation, chemical properties, and its practical … Read more