CO2 Lewis Structure
If you’ve ever wondered about the chemical makeup of carbon dioxide or needed to understand its bonding for a chemistry class, you’re in the right place. In this blog post, we’ll break down the CO₂ Lewis structure in a way that’s easy to grasp, making this essential chemistry concept accessible to everyone.
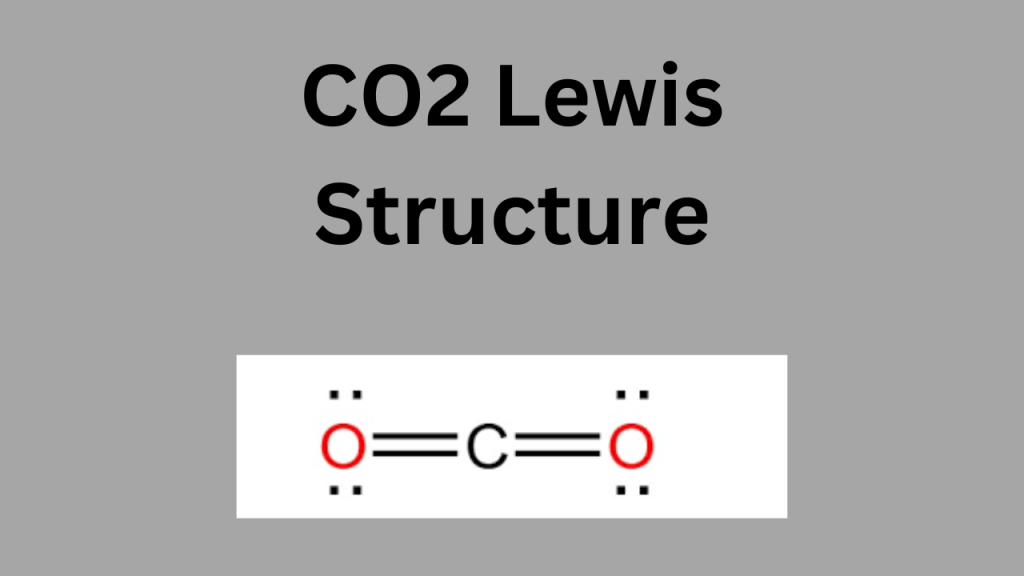
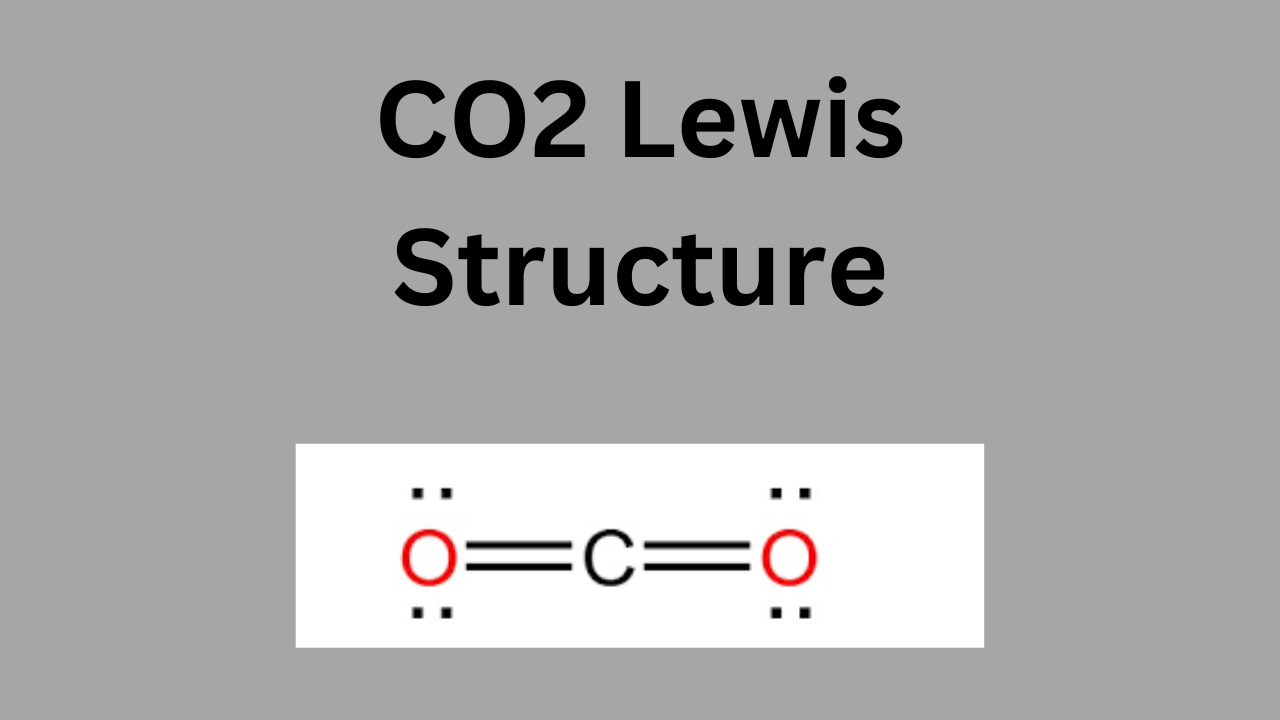
Understanding the Lewis structure of CO₂ (carbon dioxide) is essential for grasping the molecule’s chemical properties and behavior. The Lewis structure reveals that CO₂ has a linear shape with two double bonds between the carbon atom and each oxygen atom. This structure shows that CO₂ is a nonpolar molecule, which affects its solubility, reactivity, and role in processes like photosynthesis and cellular respiration. Additionally, the Lewis structure helps us understand CO₂’s role as a greenhouse gas, as its specific bonding and linear shape contribute to its ability to absorb and re-emit infrared radiation, influencing Earth’s climate. Thus, knowing the Lewis structure of CO₂ provides insight into both chemical interactions and environmental impacts.
If you have any question regarding H2O2 Lewis structure we have a detailed blog post on it.
What is a Lewis Structure?
Before we dive into the specifics of CO₂, let’s briefly discuss what a Lewis structure is. Named after Gilbert N. Lewis, a Lewis structure is a diagram that shows the bonding between atoms of a molecule and the lone pairs of electrons that may exist. It’s a handy way to visualize how atoms share electrons and form bonds.
Why is the CO₂ Lewis Structure Important?
Carbon dioxide (CO₂) is a crucial molecule in various fields, from environmental science to biochemistry. Understanding its Lewis structure helps in comprehending its chemical properties, reactions, and role in processes like photosynthesis and respiration.
Step-by-Step Guide to Drawing the CO₂ Lewis Structure
Let’s break down the process of drawing the Lewis structure for CO₂.
Step 1: Determine the Total Number of Valence Electrons
Carbon (C) has 4 valence electrons, and each oxygen (O) atom has 6 valence electrons. Since there are two oxygen atoms, we multiply 6 by 2.
4 (C)+6×2 (O)=16 valence electrons4 \text{ (C)} + 6 \times 2 \text{ (O)} = 16 \text{ valence electrons}4 (C)+6×2 (O)=16 valence electrons
Step 2: Choose the Central Atom
Carbon is less electronegative than oxygen, so it will be the central atom. The structure will have carbon in the middle with oxygen atoms on each side.
Step 3: Draw Single Bonds
Start by drawing single bonds between the carbon and each oxygen atom. Each single bond uses 2 electrons.
O−C−O\text{O} – \text{C} – \text{O}O−C−O
This uses up 4 of the 16 valence electrons, leaving us with 12 electrons.
Step 4: Complete the Octets of the Outer Atoms
Next, place the remaining electrons around the oxygen atoms to complete their octets. Each oxygen atom needs 8 electrons to be stable, but they already have 2 from the single bond.
Placing 6 more electrons around each oxygen:
O:2+6=8\text{O} : 2 + 6 = 8O:2+6=8
This uses up all 12 of the remaining electrons:
O(6)−C(4)−O(6)\text{O} (6) – \text{C} (4) – \text{O} (6)O(6)−C(4)−O(6)
Step 5: Form Double Bonds
Carbon needs 8 electrons to complete its octet, but currently has only 4 from the single bonds. We need to form double bonds between carbon and each oxygen.
So, we take two lone pairs from each oxygen and form double bonds:
O=C=O\text{O} = \text{C} = \text{O}O=C=O
Now, carbon shares 8 electrons (4 from each double bond), and each oxygen also has 8 electrons (4 from the double bond and 4 from lone pairs).
Step 6: Verify the Structure
Ensure that all atoms have complete octets:
- Carbon has 8 electrons (satisfied by the two double bonds).
- Each oxygen has 8 electrons (4 in the double bond and 4 as lone pairs).
Visual Representation
Here’s a visual representation of the CO₂ Lewis structure:
mathematicaCopy code O=C=O
Conclusion
Understanding the CO₂ Lewis structure is a fundamental skill in chemistry that reveals much about the molecule’s stability and reactivity. By following the steps outlined above, you can confidently draw and interpret the Lewis structure of carbon dioxide. This foundational knowledge not only aids in academic pursuits but also enhances our comprehension of critical processes affecting our environment and life on Earth.
4 Comments