CS Lewis Structure
The CS Lewis structure represents how carbon and sulfur atoms share electrons to form bonds. In this molecule, carbon is bonded to sulfur through a triple bond, making it a linear molecule. The CS molecule is important in various chemical reactions, especially in organic and inorganic chemistry, and it is used in the synthesis of several compounds. Understanding the Lewis structure of CS is crucial for predicting its reactivity, stability, and behavior in different chemical environments.
Step-by-Step Guide to Drawing the CS Lewis Structure
To draw the CS Lewis structure, follow these steps:
1. Count the Total Valence Electrons
Each atom in the molecule contributes electrons from its outermost shell (valence electrons):
- Carbon (C): Carbon, being in Group 14 of the periodic table, has 4 valence electrons.
- Sulfur (S): Sulfur, being in Group 16, has 6 valence electrons.
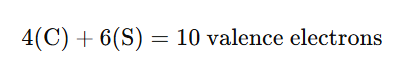
2. Determine the Central Atom
In the CS Lewis structure, carbon (C) is the central atom. This is because carbon typically forms covalent bonds, and it is less electronegative than sulfur, making it the more likely central atom in the molecule.
3. Form the Bonds
Place the sulfur (S) atom and the carbon (C) atom close to each other. Carbon will form a triple bond with sulfur, as sulfur can accommodate more bonding pairs in its valence shell. This triple bond involves six electrons (two pairs) being shared between carbon and sulfur. After forming the triple bond, both carbon and sulfur will have complete valence shells.
4. Distribute the Electrons
After creating the triple bond, you have 6 electrons shared between the two atoms. The remaining 4 electrons will be placed as lone pairs on the sulfur atom to complete its octet. Carbon has already completed its octet by sharing electrons with sulfur.
5. Check for Formal Charges
In the CS Lewis structure, both carbon and sulfur should ideally have formal charges of zero. To check this, calculate the formal charge on each atom:
- Carbon: Carbon has 4 valence electrons and shares 6 electrons in bonds (3 bonds × 2 electrons each), giving it a formal charge of zero.
- Sulfur: Sulfur has 6 valence electrons and shares 6 electrons in bonds (triple bond with carbon), plus it has two lone pairs of electrons. This also gives sulfur a formal charge of zero.
Key Features of the CS Lewis Structure
- Bonding: The CS molecule is held together by a triple bond between carbon and sulfur. This is a strong bond that requires the sharing of six electrons (three pairs) between the two atoms.
- Electron Distribution: The electrons are evenly distributed between the carbon and sulfur atoms, with carbon fulfilling the octet rule through bonding with sulfur and sulfur completing its octet with the triple bond and lone pairs.
- Geometry: The CS molecule has a linear geometry. This is because the triple bond between carbon and sulfur is a straight line, and there are no lone pairs on the central carbon atom to distort the structure. The bond angle between the carbon and sulfur atoms is approximately 180°.
- Formal Charges: Both the carbon and sulfur atoms have formal charges of zero, indicating that the structure is stable and that the bonding is optimal.
Chemical Properties of CS
The CS Lewis structure helps explain several important chemical properties of the carbon monosulfide molecule:
- Reactivity: The triple bond in CS makes it reactive in various chemical reactions, particularly with other molecules that can break the strong triple bond. CS can react with other compounds to form different sulfur and carbon-based molecules.
- Bond Strength: The triple bond between carbon and sulfur is quite strong, which gives CS a relatively high bond dissociation energy. This makes the molecule stable, but it can still undergo reactions under the right conditions.
- Industrial Uses: While CS is not as commonly encountered as other sulfur or carbon compounds, it has applications in the synthesis of chemicals like thioketones, and it is a component in certain industrial reactions involving carbon and sulfur.
Common Questions About the CS Lewis Structure
1. What is the Bonding in CS?
The bonding in CS involves a triple bond between the carbon and sulfur atoms. This triple bond consists of one sigma bond and two pi bonds, which are shared between the two atoms to form a stable structure.
2. What is the Geometry of the CS Molecule?
The CS molecule has a linear geometry. The two atoms (carbon and sulfur) are arranged in a straight line with an approximate bond angle of 180°.
3. How Many Lone Pairs Are on the Sulfur Atom in CS?
In the CS Lewis structure, sulfur has two lone pairs of electrons. These lone pairs are essential for completing sulfur’s octet, as it shares three pairs of electrons with carbon through the triple bond.
4. Is CS Polar or Nonpolar?
The CS molecule is polar due to the difference in electronegativity between carbon and sulfur. While the linear geometry of the molecule might suggest a nonpolar nature, the bond between carbon and sulfur is polar due to sulfur being more electronegative than carbon, creating a dipole moment.
Conclusion
The CS Lewis structure is a simple yet important representation of the bonding and electron distribution in carbon monosulfide. The molecule consists of a strong triple bond between carbon and sulfur, and the linear geometry and the proper electron distribution contribute to its stability. By understanding the Lewis structure, we can better predict the molecule’s reactivity and its behavior in chemical reactions. Whether you are studying organic or inorganic chemistry, the CS Lewis structure is essential for understanding the interactions between carbon and sulfur in various chemical processes.
One Comment