Fe2S Lewis Structure
Fe₂S Lewis Structure: A Comprehensive Guide
The Fe₂S Lewis structure represents the bonding and electron distribution in a molecule composed of two iron (Fe) atoms and one sulfur (S) atom. This structure is essential for understanding how the atoms interact and how the molecule behaves chemically. In this guide, we will explore how to draw the Fe₂S Lewis structure, its key features, and its significance in chemistry.
What is the Fe₂S Lewis Structure?
The Fe₂S Lewis structure refers to how two iron atoms are bonded to a single sulfur atom, where iron typically exists in a positive oxidation state. The structure is important in understanding the bonding in iron sulfide, which is a compound that can occur in different forms, such as FeS or Fe₂S, depending on the stoichiometry. The Fe₂S compound can be seen as a metal-metal bond between iron atoms and a metal-nonmetal bond with sulfur. In many cases, the Fe₂S structure is part of larger minerals or used industrially in various reactions, such as in metallurgy.
Step-by-Step Guide to Drawing the Fe₂S Lewis Structure
To draw the Fe₂S Lewis structure, we need to follow these key steps:
1. Count the Total Valence Electrons
Each atom in the molecule contributes electrons from its outermost shell (valence electrons):
- Iron (Fe): Iron is a transition metal and has an electron configuration of [Ar] 3d⁶ 4s². For the purpose of bonding, each iron atom contributes 2 valence electrons.
- Sulfur (S): Sulfur is in Group 16 of the periodic table, so it has 6 valence electrons.
For Fe₂S, the total number of valence electrons is:
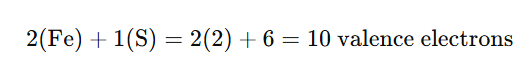
2. Determine the Central Atom
In the Fe₂S structure, sulfur (S) is the nonmetal and will be at the center of the structure, bonded to both iron atoms. Iron is a metal, and metals like to form bonds with nonmetals, so sulfur will be surrounded by the iron atoms.
3. Form the Bonds
Place the iron atoms on either side of the sulfur atom and connect them with single bonds. Each iron atom will bond to sulfur through a single bond, sharing electrons.
4. Distribute the Electrons
Now that you’ve placed the bonds, you need to distribute the remaining electrons to ensure that each atom has a complete electron configuration:
- Each iron atom has already used its two valence electrons in bonding to sulfur, so the remaining electrons will go to sulfur.
- Sulfur will have lone pairs of electrons around it to complete its octet.
The Fe-S bond will typically be a single bond, but in some cases, the bonding can involve delocalization or other factors based on the compound’s specific form and oxidation states of iron.
5. Check Formal Charges
In the Fe₂S Lewis structure, iron atoms will have formal charges, as iron usually forms bonds in a higher oxidation state (typically +2 or +3). In this case, the formal charges on the iron atoms may not be zero, depending on the oxidation state of iron. However, the sulfur atom will likely have a formal charge close to zero, given that sulfur follows the octet rule.
Key Features of the Fe₂S Lewis Structure
- Bonding: The Fe₂S molecule consists of two iron atoms bonded to a sulfur atom through single bonds. The bonding is characterized by the electron transfer or sharing between the metal and nonmetal atoms.
- Electron Distribution: Each iron atom shares two electrons with sulfur, resulting in a bond. Sulfur, with six valence electrons, typically completes its octet by gaining electrons or forming bonds with metals.
- Oxidation States: Iron in Fe₂S usually exists in the +2 or +3 oxidation state, which affects the bonding and formal charges in the structure. This is due to the electron loss by iron to form positive ions.
- Molecular Geometry: The Fe₂S molecule has a linear geometry with sulfur at the center and iron atoms at the ends. The iron atoms are typically sp hybridized in this case, forming linear bonds with sulfur.
Chemical and Physical Properties of Fe₂S
The Fe₂S Lewis structure helps explain several important properties of iron sulfide compounds:
- Magnetism: Fe₂S, like other iron-based compounds, can exhibit magnetic properties due to the presence of iron ions in various oxidation states.
- Reactivity: Iron sulfide is reactive with acids and can release hydrogen sulfide gas (H₂S) when reacting with certain substances.
- Industrial Importance: Iron sulfide is commonly found in natural minerals, and its structure plays a significant role in various industrial processes, including metal extraction and chemical manufacturing.
Common Questions About the Fe₂S Lewis Structure
1. What Is the Bonding in Fe₂S?
The bonding in Fe₂S involves iron atoms sharing electrons with sulfur through single bonds. Iron can form bonds in different oxidation states, typically +2 or +3, which affects the bonding nature. The bonding in Fe₂S is mainly ionic, with some covalent character due to electron sharing between iron and sulfur.
2. What is the Geometry of Fe₂S?
The geometry of Fe₂S is linear, with sulfur at the center and two iron atoms on either side, forming 180° bond angles. This is typical for molecules with two atoms bonded to a central atom and no lone pairs on the central atom.
3. How Many Lone Pairs Are on the Sulfur Atom in Fe₂S?
In the Fe₂S Lewis structure, sulfur typically has two lone pairs of electrons. These lone pairs are necessary for sulfur to complete its octet and maintain stability in the molecule.
4. Is Fe₂S Polar or Nonpolar?
The Fe₂S molecule is nonpolar due to its linear geometry. The dipoles in the Fe-S bonds cancel each other out, resulting in a nonpolar molecule. This makes Fe₂S relatively stable in nonpolar environments.
Conclusion
The Fe₂S Lewis structure is an essential representation of the bonding and electron distribution in iron disulfide. By understanding how to draw this structure, we can gain insight into the molecule’s properties, reactivity, and role in various industrial and chemical processes. Iron sulfide is an important compound in metallurgy and chemical reactions, and its unique structure makes it valuable in a variety of applications. Whether it’s used for its magnetic properties or its role in chemical reactions, understanding Fe₂S’s bonding helps explain its behavior in both nature and industry.
One Comment