How To Identify Saturation In Organic Compounds
In organic chemistry, determining whether a compound is saturated or unsaturated is crucial for understanding its chemical behavior, stability, and reactivity. Saturation in organic compounds refers to the presence or absence of double and triple bonds in their molecular structure. This guide will help you learn how to identify the saturation level of an organic compound using several key methods and indicators.
What Does Saturation Mean in Organic Chemistry?
An organic compound is considered saturated if all the carbon-carbon bonds in the molecule are single bonds. Saturated compounds have the maximum number of hydrogen atoms bonded to carbon, meaning no additional atoms can attach without breaking a bond. A classic example of a saturated compound is alkanes (e.g., methane, ethane, propane).
Conversely, unsaturated compounds contain one or more double (alkenes) or triple (alkynes) bonds between carbon atoms, which reduces the number of hydrogen atoms attached to the molecule. Examples include ethene (C₂H₄) and ethyne (C₂H₂).
Methods to Identify Saturation in Organic Compounds
To determine if a compound is saturated or unsaturated, chemists rely on several techniques:
1. Examine the Molecular Formula
One of the quickest ways to assess saturation is by analyzing the molecular formula:
- For alkanes (saturated hydrocarbons), the general formula is CₙH₂ₙ₊₂. If the compound fits this formula, it’s likely saturated.
- Alkenes (unsaturated hydrocarbons with one double bond) have the formula CₙH₂ₙ, while alkynes (one triple bond) have CₙH₂ₙ₋₂.
For example, C₂H₆ (ethane) fits the formula for alkanes, suggesting it’s saturated, while C₂H₄ (ethylene) suggests the presence of a double bond and is unsaturated.
2. Look for Double or Triple Bonds in the Structure
The presence of double (C=C) or triple (C≡C) bonds in the molecular structure indicates unsaturation:
- Double bonds are typically found in alkenes, while triple bonds are common in alkynes.
- Visualizing the structure using Lewis structures or line diagrams can help identify these bonds easily.
For instance, benzene (C₆H₆) has alternating single and double bonds in a ring structure, indicating it’s an unsaturated aromatic compound.
3. Use the Degree of Unsaturation (DU) Formula
The degree of unsaturation formula helps calculate the number of rings, double bonds, or triple bonds in a compound based on its molecular formula
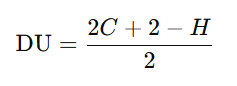
Where:
- C is the number of carbon atoms
- H is the number of hydrogen atoms
For instance, if a compound has a DU of 1, it may contain either one double bond or one ring structure, indicating unsaturation. A DU of 0 indicates that the compound is likely saturated.
4. Bromine Test for Unsaturation
The bromine test is a simple chemical test to identify unsaturation:
- In this test, bromine (Br₂), a reddish-brown liquid, is added to the compound in question. If the solution’s color quickly fades, it indicates a reaction with double or triple bonds, confirming unsaturation.
- Saturated compounds, which lack double or triple bonds, do not react with bromine, so the solution retains its color.
This test is often used in laboratories to confirm the presence of alkenes or alkynes.
5. Infrared (IR) Spectroscopy
IR spectroscopy is a more precise method to detect saturation and unsaturation:
- Saturated compounds display characteristic C-H stretching vibrations in the 2800–3000 cm⁻¹ region.
- Unsaturated compounds show additional peaks due to C=C stretching (around 1640 cm⁻¹) for alkenes or C≡C stretching (around 2100–2260 cm⁻¹) for alkynes.
IR spectroscopy allows for a detailed analysis of the bond types in the molecule, which helps confirm the saturation level.
Practical Examples of Identifying Saturation
- Cyclohexane (C₆H₁₂):
- Formula does not follow CₙH₂ₙ₊₂, indicating potential unsaturation.
- The structure reveals it has a ring with only single bonds, making it saturated.
- Hexene (C₆H₁₂):
- Follows CₙH₂ₙ, suggesting the presence of a double bond.
- This compound is unsaturated due to the double bond in its structure.
- Acetylene (C₂H₂):
- Follows CₙH₂ₙ₋₂, typical for alkynes.
- The triple bond makes it unsaturated.
Why Identifying Saturation is Important
Determining the saturation of organic compounds is essential for:
- Predicting Reactivity: Unsaturated compounds are more reactive than saturated ones because of the presence of double or triple bonds, which are less stable than single bonds.
- Understanding Physical Properties: Saturated compounds are often more stable and have higher melting and boiling points than their unsaturated counterparts.
- Industrial Applications: Many organic reactions, including hydrogenation and polymerization, rely on the reactivity of unsaturated compounds to create products like plastics, fuels, and pharmaceuticals.
Conclusion
Identifying saturation in organic compounds involves analyzing the molecular formula, examining structural bonds, using the degree of unsaturation, and performing tests like the bromine test or IR spectroscopy. By following these steps, you can accurately determine whether an organic compound is saturated or unsaturated, which has significant implications in both theoretical and applied chemistry.