How To Trace Lewis Structure
Lewis structures are essential tools in chemistry for visualizing the bonding between atoms and the arrangement of electrons in molecules. They help us understand molecular geometry, bond strength, and even predict reactivity. Tracing a Lewis structure involves representing a molecule’s atoms, bonds, and lone pairs of electrons in a simple diagram. Here’s a step-by-step guide to accurately trace a Lewis structure.
Step 1: Determine the Total Valence Electrons
The first step is to count the total number of valence electrons for all atoms in the molecule.
- Look at each atom in the molecule, and note the number of valence electrons it has, which corresponds to its group number in the periodic table.
- Add up the valence electrons of each atom to get the total for the molecule.
For example, in H₂O:
- Oxygen has 6 valence electrons.
- Hydrogen has 1 valence electron each, so 2 × 1 = 2 for two hydrogens.
- The total valence electrons = 6 + 2 = 8.
Step 2: Choose the Central Atom
The central atom is typically the least electronegative atom (except for hydrogen, which is always on the outside). The central atom is where other atoms are connected.
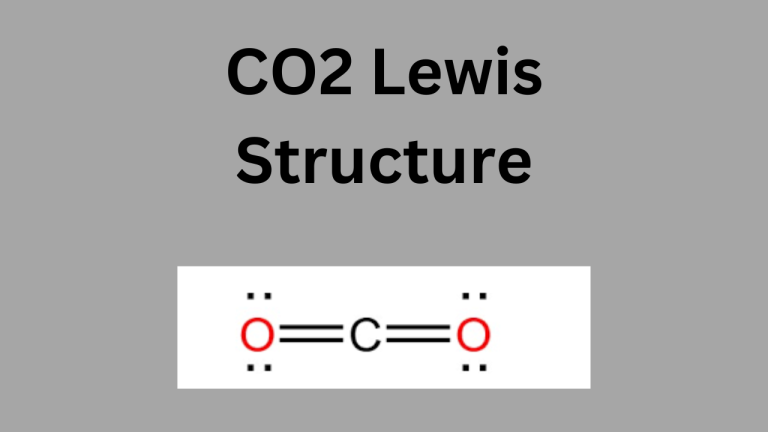
For example, in CO₂ (carbon dioxide):
- Carbon (C) is less electronegative than oxygen (O), so carbon is the central atom.
Step 3: Connect the Atoms with Single Bonds
Draw single bonds between the central atom and the surrounding atoms. Each single bond represents two electrons shared between atoms.
For example, in H₂O:
- Draw a single bond between oxygen and each hydrogen atom.
At this point, the structure would look like this:
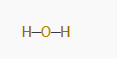
Step 4: Distribute Remaining Electrons as Lone Pairs
After drawing single bonds, distribute the remaining electrons around the atoms to satisfy the octet rule (or duet rule for hydrogen).
- Start by placing lone pairs around the outer atoms (except hydrogen, which only needs two electrons).
- Then, place any remaining electrons on the central atom.
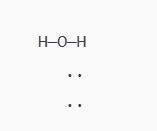
In H₂O:
- After the two single bonds, you’ve used 4 electrons, leaving 4 more.
- Place the remaining 4 electrons as lone pairs on oxygen to complete its octet.
So, H₂O now looks like this:
Step 5: Check for Octet Rule Fulfillment
Ensure that each atom (other than hydrogen) has eight electrons around it. This includes electrons in bonds and lone pairs. If any atom doesn’t meet the octet rule, consider using double or triple bonds.
For example, in CO₂:
- After initially drawing single bonds, carbon will not have a full octet.
- To fulfill the octet, change each single bond to a double bond, creating the final structure: O=C=O.
Step 6: Calculate Formal Charges (Optional but Recommended)
Formal charges help confirm that the most stable structure has been drawn. The goal is to minimize formal charges, ideally bringing each atom’s formal charge to zero or close to zero.
The formula for calculating formal charge on an atom is:Formal Charge=Valence Electrons−(Lone Pair Electrons+Bonding Electrons2)\text{Formal Charge} = \text{Valence Electrons} – (\text{Lone Pair Electrons} + \frac{\text{Bonding Electrons}}{2})Formal Charge=Valence Electrons−(Lone Pair Electrons+2Bonding Electrons)
Check the formal charge for each atom, adjusting bonds if needed to achieve the lowest possible charge.
Step 7: Draw the Final Lewis Structure
Based on your adjustments, draw the final structure with all bonds and lone pairs shown. This structure will represent the most stable form of the molecule.
Example: Tracing the Lewis Structure for NH₃ (Ammonia)
- Count Valence Electrons:
- Nitrogen (N) has 5 valence electrons.
- Each Hydrogen (H) has 1 valence electron, totaling 3 for the three hydrogens.
- Total electrons = 5 + 3 = 8.
- Choose the Central Atom:
- Nitrogen is the central atom because hydrogen can only bond to one atom.
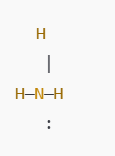
- Draw Single Bonds:
- Draw single bonds between nitrogen and each hydrogen.
- Distribute Remaining Electrons:
- After the single bonds, 6 electrons are used, leaving 2.
- Place the remaining 2 electrons as a lone pair on nitrogen.
- Check for Octet Rule:
- Nitrogen now has an octet (3 bonds + 1 lone pair), and each hydrogen has 2 electrons, fulfilling the duet rule.
- Calculate Formal Charge:
- Nitrogen and hydrogen atoms have a formal charge of zero, making this structure stable.
The final NH₃ Lewis structure looks like this:
Conclusion
Tracing a Lewis structure involves counting valence electrons, arranging atoms, forming bonds, distributing lone pairs, and verifying the octet rule. By following these steps, you can accurately draw Lewis structures, which serve as invaluable tools in visualizing molecular geometry and predicting chemical behavior.