NH3 Lewis Structure
Drawing Lewis structures is fundamental in chemistry to understand how molecules form and behave. This guide will walk you through the NH3 (Ammonia) Lewis structure step-by-step and explain its geometry, polarity, bond angles, and much more.
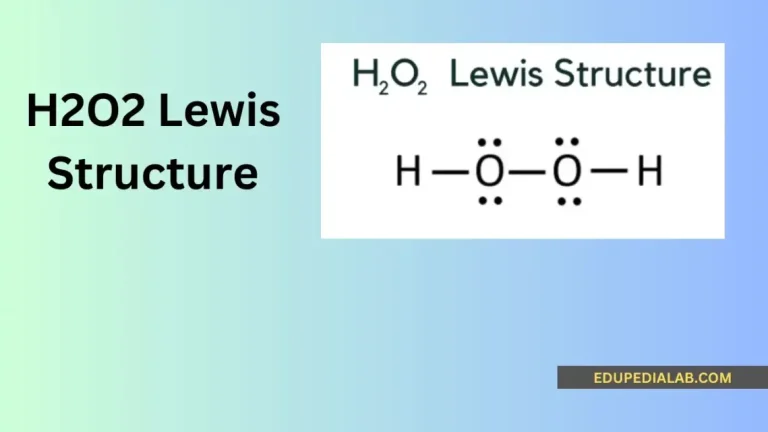
Do you know that H2O2 is a very unique molecule when it comes to drawing lewis structure?
What is the Lewis Structure?
Definition of Lewis Structure
A Lewis structure is a visual representation of the bonds between atoms and the lone pairs of electrons in a molecule. By illustrating these, chemists can predict molecular shapes and reactivity.
Importance of Lewis Structures in Chemistry
Lewis structures help us understand how atoms connect and the arrangement of electrons, which influences molecular behavior, stability, and reactions.
Basic Characteristics of Ammonia (NH3)
Molecular Composition of Ammonia
Ammonia consists of one nitrogen (N) atom and three hydrogen (H) atoms. Its chemical formula, NH3, signifies this composition.
Physical and Chemical Properties of NH3
Ammonia is a colorless gas with a pungent smell, commonly found in cleaning solutions and fertilizers. Its molecular properties are key to understanding its interactions and applications.
Steps to Draw the Lewis Structure of NH3
Step 1: Count the Valence Electrons
Nitrogen has five valence electrons, and each hydrogen atom has one. Adding them up, we have 5 (from N) + 3 (from H) = 8 valence electrons.
Step 2: Identify the Central Atom
The nitrogen atom serves as the central atom since it is less electronegative than hydrogen and can bond with multiple atoms.
Step 3: Form Single Bonds Between Nitrogen and Hydrogen Atoms
Connect the nitrogen atom to each of the three hydrogen atoms with a single bond, using up six of the eight valence electrons.
Step 4: Place Remaining Electrons on the Nitrogen Atom
After bonding, two electrons remain, which form a lone pair on nitrogen.
Step 5: Check Octet Rule and Stability
Nitrogen has an octet with three bonds and one lone pair, making the structure stable and satisfying the octet rule.
Explanation of the NH3 Lewis Structure
Bonding in NH3
Each hydrogen atom shares one electron with nitrogen, forming three N–H bonds. These single bonds are covalent, meaning electrons are shared between atoms.
Molecular Geometry of NH3 (Trigonal Pyramidal)
NH3 has a trigonal pyramidal shape due to its lone pair of electrons on nitrogen, which pushes the three hydrogen atoms down.
Lone Pair and Bond Pair Interaction
The lone pair on nitrogen causes a repulsion with the bonding pairs, giving NH3 its pyramidal structure rather than a flat, triangular shape.
Polarity of NH3
What is Molecular Polarity?
Polarity in molecules occurs when there is an unequal distribution of electrons, causing a molecule to have a partial positive and partial negative side.
How NH3’s Structure Affects its Polarity
NH3 is a polar molecule because the lone pair on nitrogen creates an asymmetrical shape, causing a dipole moment and resulting in a polar molecule.
Bond Angles in NH3
Typical Bond Angle in NH3
The bond angle in NH3 is approximately 107 degrees, slightly less than the 109.5 degrees expected in a perfect tetrahedral shape.
Effect of Lone Pair on Bond Angle
The lone pair on nitrogen pushes the hydrogen atoms closer together, reducing the bond angle slightly to 107 degrees.
Electron and Molecular Geometry of NH3
VSEPR Theory Overview
Valence Shell Electron Pair Repulsion (VSEPR) theory explains that electron pairs around a central atom will position themselves to minimize repulsion, affecting the molecular shape.
Electron Geometry vs. Molecular Geometry
The electron geometry of NH3 is tetrahedral due to four electron regions, but its molecular geometry is trigonal pyramidal because only three of these are bonding pairs.
Hybridization of NH3
What is Hybridization?
Hybridization is the concept of atomic orbitals merging to form new orbitals, which impacts molecular shape and bonding.
Hybridization in Ammonia (NH3)
In NH3, the nitrogen atom undergoes sp3 hybridization, combining one s orbital and three p orbitals to form a structure supporting the three N–H bonds and a lone pair.
Applications and Importance of NH3
Uses of Ammonia in Various Industries
Ammonia is essential in agriculture (as fertilizer), cleaning products, and the chemical industry.
Role of NH3 in Environmental Chemistry
Ammonia plays a significant role in nitrogen cycles and can impact air quality, making it important in environmental chemistry.
Conclusion
The Lewis structure of NH3 provides insight into its geometry, bonding, polarity, and more. By understanding these aspects, we can appreciate ammonia’s chemical properties and its relevance in various applications. With a trigonal pyramidal shape, NH3 is a polar molecule with intriguing molecular properties, serving as an excellent example of how the arrangement of atoms and electrons influences molecular behavior.
FAQs
Why does NH3 have a trigonal pyramidal shape?
NH3 has a trigonal pyramidal shape due to the lone pair on nitrogen, which repels the hydrogen atoms, pushing them downward.
Is NH3 polar or nonpolar?
NH3 is polar because its asymmetrical shape creates a dipole moment.
How many lone pairs are on the nitrogen atom in NH3?
There is one lone pair on the nitrogen atom in NH3.
What is the bond angle in NH3?
The bond angle in NH3 is approximately 107 degrees, slightly less than the typical tetrahedral angle.
How is the Lewis structure helpful in understanding NH3’s properties?
The Lewis structure reveals bonding, electron arrangement, and polarity, helping to predict NH3’s chemical behavior.
2 Comments