O3 Chemical Reactivity
Ozone (O₃) is a highly reactive molecule consisting of three oxygen atoms. Known for its role in the Earth’s atmosphere, particularly in protecting us from harmful ultraviolet (UV) radiation, ozone also plays a significant role in various chemical processes. Understanding O₃ chemical reactivity is essential for fields ranging from environmental science to industrial chemistry. In this guide, we will explore the factors that influence the reactivity of ozone, how it interacts with different substances, and the key chemical reactions it undergoes.
Do you want to know if ozone is polar or non polar check our recent detailed blog post
What is O₃ Chemical Reactivity?
The chemical reactivity of O₃ refers to the way ozone molecules interact with other chemicals, often resulting in the formation of new compounds. Ozone is a powerful oxidizing agent, meaning it readily accepts electrons from other substances, which can lead to significant chemical transformations. Because of its highly reactive nature, O₃ can break bonds in molecules, making it crucial in both beneficial and harmful reactions.
Factors Affecting the Chemical Reactivity of O₃
Several factors influence the reactivity of O₃, including its molecular structure, the presence of other reactive species, and the environmental conditions under which reactions take place. Ozone’s reactivity can be described by the following key elements:
1. Bonding and Molecular Structure
Ozone consists of three oxygen atoms bonded in a bent shape, with one single bond and one double bond between the oxygen atoms. This structure makes O₃ a relatively unstable molecule, as the single bond is weaker and more prone to breaking. The instability of the O–O bond leads to the molecule’s high reactivity, making ozone a powerful oxidizing agent.
2. Electronegativity of Oxygen
Oxygen is highly electronegative, meaning it strongly attracts electrons. When ozone interacts with other molecules, it can easily accept electrons, creating reactive oxygen species (ROS) that are involved in oxidation reactions. This property allows ozone to react with a wide variety of organic and inorganic compounds.
3. Environmental Conditions
The reactivity of ozone can vary depending on temperature, pressure, and the presence of other gases or molecules. For instance, ozone is more reactive at higher temperatures, and its reactivity is influenced by the concentration of ozone in the surrounding environment. The presence of pollutants or other chemical species can also alter the speed and outcomes of ozone reactions.
Key Chemical Reactions Involving O₃
Ozone participates in numerous chemical reactions in both the atmosphere and industrial applications. Here are some important examples:
1. Ozone Depletion in the Stratosphere
One of the most well-known reactions involving ozone is its breakdown in the stratosphere, particularly in the presence of chlorofluorocarbons (CFCs). Ozone reacts with chlorine atoms, leading to the destruction of the ozone layer. This process can be described by the following reaction:
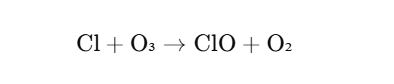
In this reaction, chlorine atoms break down ozone molecules, contributing to the thinning of the ozone layer.
2. Reaction with Nitrogen Oxides (NOₓ)
Ozone also reacts with nitrogen oxides (NOₓ), which are pollutants produced by vehicle emissions and industrial processes. These reactions can lead to the formation of nitrogen dioxide (NO₂), which is involved in the formation of smog. One such reaction is:
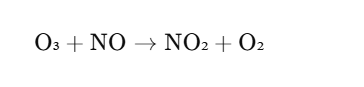
This reaction is a key process in the atmospheric chemistry of smog and pollution.
3. Ozone in Water Treatment
In water treatment, ozone is used to disinfect water and break down organic pollutants. Ozone reacts with bacteria, viruses, and organic matter, effectively sterilizing and purifying water. The reaction is typically as follows:
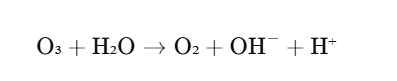
Here, ozone decomposes in the presence of water to form hydroxyl radicals (OH⁻), which are highly reactive and capable of breaking down contaminants.
Common Questions About O₃ Chemical Reactivity
1. What Makes Ozone a Powerful Oxidizing Agent?
Ozone is a powerful oxidizing agent due to its molecular structure and the electronegativity of oxygen. The unstable O–O bond makes ozone readily reactive, and its ability to accept electrons allows it to easily oxidize other substances.
2. How Does O₃ React with Organic Compounds?
Ozone reacts with organic compounds by breaking carbon-carbon bonds, leading to the formation of smaller molecules or oxidized products. This reaction is often used in industrial applications like ozonolysis, where ozone is used to break down alkenes into aldehydes and ketones.
3. What Are the Environmental Impacts of O₃ Chemical Reactivity?
While ozone is beneficial in the stratosphere, where it protects the Earth from UV radiation, its chemical reactivity in the lower atmosphere can contribute to air pollution. Ground-level ozone is a key component of smog and can harm human health by irritating the respiratory system and contributing to asthma and other conditions.
4. How Is Ozone Used in Industrial Applications?
Ozone’s reactivity makes it valuable in industrial applications such as water treatment, air purification, and chemical manufacturing. Its ability to break down contaminants and sterilize surfaces makes it an effective tool for improving public health and safety in various sectors.
Conclusion
The chemical reactivity of O₃ is a fundamental aspect of its behavior, both in the atmosphere and in various chemical processes. Ozone’s ability to act as a powerful oxidizing agent allows it to play a crucial role in environmental chemistry, industrial applications, and even air quality control. Whether breaking down pollutants or contributing to the destruction of the ozone layer, understanding O₃’s reactivity helps us appreciate its dual nature—both beneficial and potentially harmful depending on its location and concentration.
By understanding how ozone interacts with other substances, we gain insight into both its positive uses, like water treatment, and its negative effects, such as ozone depletion and pollution. Mastering the chemical reactivity of O₃ allows chemists, environmental scientists, and industry professionals to better harness its potential while mitigating its risks.
One Comment