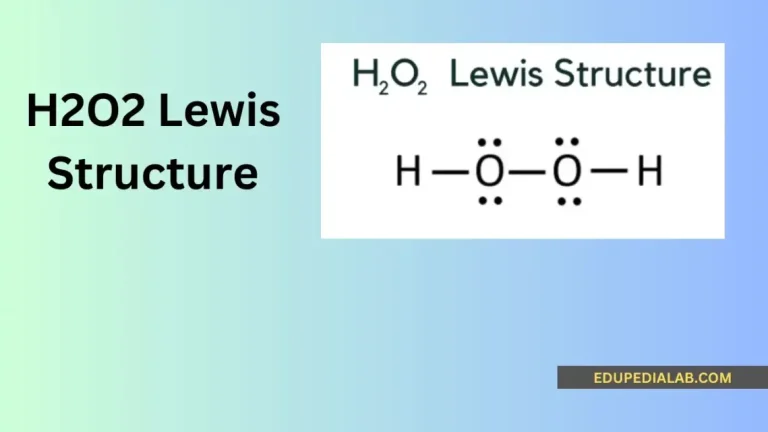
H2O2 Lewis Structure
The Hydrogen Peroxide (H2O2) is one of the volatile, viscous The world of chemistry unfolds its secrets through the intricate dance of atoms and bonds. One such fascinating compound is hydrogen peroxide, and at the heart of its understanding lies the H2O2 Lewis structure. You can watch this video to have more information regarding the…