PCl3 Electronegativity Calculation
Phosphorus trichloride (PCl₃) is a covalent compound consisting of a phosphorus (P) atom bonded to three chlorine (Cl) atoms. Understanding the electronegativity values of the atoms involved helps us predict the bond polarity and molecular properties of PCl₃. In this guide, we’ll look at how to calculate the bond polarity of PCl₃ based on electronegativity differences and analyze the molecule’s overall polarity.
Step 1: Electronegativity of Phosphorus and Chlorine
Electronegativity is the tendency of an atom to attract electrons in a chemical bond. Each element has an electronegativity value on the Pauling scale:
- Phosphorus (P) has an electronegativity of approximately 2.19.
- Chlorine (Cl) has a higher electronegativity, around 3.16.
The difference in these values will help determine the type and polarity of the bonds in PCl₃.
Step 2: Calculating the Electronegativity Difference
The electronegativity difference (ΔEN) between bonded atoms indicates bond polarity
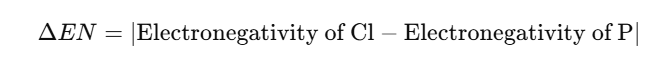
For each P-Cl bond
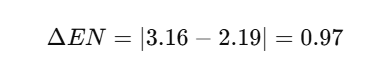
This difference of 0.97 suggests that each P-Cl bond is polar covalent, meaning electrons are not equally shared but are more attracted to the chlorine atom due to its higher electronegativity.
Step 3: Assessing Molecular Polarity in PCl₃
Although each P-Cl bond is polar, it’s important to analyze the molecule’s shape to determine if it has an overall dipole moment. PCl₃ has a trigonal pyramidal molecular geometry due to the lone pair on the phosphorus atom. This shape results in an asymmetrical electron distribution, creating a net dipole moment for the molecule.
Since the electron density is pulled more towards the chlorine atoms, the overall molecule is polar. The asymmetry caused by the lone pair on phosphorus leads to a partial negative charge near the chlorine atoms and a partial positive charge near the phosphorus atom.
Key Points
- Electronegativity of P = 2.19, Cl = 3.16.
- Bond polarity: Each P-Cl bond is polar covalent, with a ΔEN of 0.97.
- Molecular polarity: Due to its trigonal pyramidal shape, PCl₃ has an overall dipole moment and is a polar molecule.
Understanding the electronegativity and bond polarity of PCl₃ helps predict its chemical behavior, such as its solubility in polar solvents and reactivity with other polar compounds.