PCl3 Lewis Structure 2023
Phosphorus trichloride (PCl₃) is a chemical compound made up of one phosphorus (P) atom and three chlorine (Cl) atoms. Understanding the PCl₃ Lewis structure is essential for students and chemistry enthusiasts who are learning about molecular bonding, electron configuration, and the principles of covalent bonding. In this guide, we will take you through the step-by-step process of drawing the Lewis structure for PCl₃, explain the molecular geometry, and answer some frequently asked questions related to PCl₃’s structure.
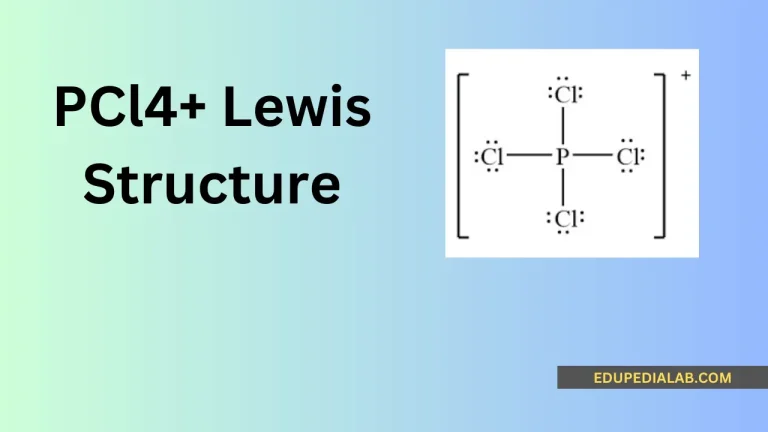
The PCl₄⁺ Lewis structure represents a positively charged phosphorus tetrachloride ion, where phosphorus is surrounded by four chlorine atoms.
What is the PCl₃ Lewis Structure?
The PCl₃ Lewis structure represents how the atoms in phosphorus trichloride are arranged and bonded. A Lewis structure is a diagrammatic representation that shows the bonding between atoms and the distribution of electrons. In the case of PCl₃, the phosphorus atom forms covalent bonds with three chlorine atoms, sharing electrons to achieve a stable electron configuration.
Steps to Draw the Lewis Structure of PCl₃
Drawing the Lewis structure of PCl₃ follows several straightforward steps. Here’s how you can draw it:
Step 1: Count the Total Valence Electrons
Each element in the molecule has a certain number of valence electrons, which are the outermost electrons involved in bonding.
- Phosphorus (P): Phosphorus is in Group 15 of the periodic table, so it has 5 valence electrons.
- Chlorine (Cl): Chlorine is in Group 17 of the periodic table, so it has 7 valence electrons. Since there are three chlorine atoms, the total number of valence electrons from chlorine is 3 × 7 = 21.
Now, add the valence electrons from phosphorus and chlorine:
- Total valence electrons = 5 (P) + 21 (Cl) = 26 valence electrons.
Step 2: Determine the Central Atom
In most molecules, the least electronegative atom tends to be the central atom. Here, phosphorus (P) is less electronegative than chlorine (Cl), so phosphorus will be the central atom in the PCl₃ molecule.
Step 3: Draw Single Bonds Between Phosphorus and Chlorine
To start drawing the structure, connect the phosphorus atom to each of the chlorine atoms with a single bond. A single bond consists of two electrons, one from each atom.
- Each P–Cl bond uses 2 electrons, so with three bonds, we use 6 electrons in total.
Step 4: Distribute the Remaining Electrons
After placing the bonds, distribute the remaining electrons as lone pairs around the atoms to satisfy the octet rule (i.e., each atom, except for hydrogen, should have 8 electrons in its valence shell).
- Phosphorus (P): After forming three bonds, phosphorus will have 6 electrons from the three single bonds. Since phosphorus can accommodate more than 8 electrons in its valence shell (due to its position in Period 3 of the periodic table), it will have 10 electrons in its valence shell.
- Chlorine (Cl): Each chlorine atom needs 8 electrons to satisfy the octet rule. Since each chlorine is already sharing 2 electrons in the single bond with phosphorus, it needs 6 more electrons. Place these electrons as lone pairs around each chlorine atom.
Now, the total number of electrons used will be 26, which matches the number of valence electrons calculated in Step 1.
Step 5: Final Structure
The final Lewis structure of PCl₃ looks like this:
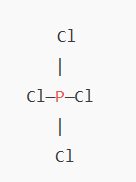
In this structure:
- The phosphorus atom is in the center, bonded to three chlorine atoms with single bonds.
- Each chlorine atom has three lone pairs of electrons, completing its octet.
- Phosphorus has 10 electrons in its valence shell, which is acceptable as it can have more than 8 electrons.
Key Characteristics of the PCl₃ Lewis Structure
- Bonding: Each P–Cl bond is a covalent bond formed by the sharing of two electrons.
- Electron Configuration: Phosphorus has 10 electrons in its valence shell, while each chlorine atom has 8 electrons.
- Geometry: The molecular geometry of PCl₃ is trigonal pyramidal due to the three bonding pairs and one lone pair of electrons on phosphorus.
- Polarity: PCl₃ is a polar molecule because of the asymmetrical shape and the difference in electronegativity between phosphorus and chlorine.
Common Questions About PCl₃ Lewis Structure
1. How to Draw the Lewis Structure of PCl₃?
To draw the Lewis structure of PCl₃, follow these steps:
- Count the total valence electrons.
- Choose phosphorus as the central atom.
- Connect phosphorus to three chlorine atoms with single bonds.
- Distribute the remaining electrons as lone pairs on the chlorine atoms, and ensure phosphorus is surrounded by 10 electrons in its valence shell.
2. What is the Best Lewis Dot Structure for PCl₃?
The best Lewis dot structure for PCl₃ is the one in which phosphorus is surrounded by 10 electrons, and each chlorine atom has 8 electrons. This configuration satisfies the valence electron requirements and adheres to the octet rule for chlorine.
3. Why is PCl₃ a Covalent Compound?
PCl₃ is a covalent compound because the phosphorus and chlorine atoms share electrons to form bonds. Phosphorus and chlorine are both nonmetals, which typically form covalent bonds through electron sharing.
4. What are the Electron Pair Arrangements in PCl₃?
In PCl₃, there are three bonding pairs and one lone pair of electrons on the phosphorus atom. The bonding pairs result from the covalent bonds with chlorine, while the lone pair contributes to the molecule’s trigonal pyramidal geometry.
Conclusion
The PCl₃ Lewis structure provides a clear representation of how phosphorus and chlorine atoms are bonded and how electrons are arranged in the molecule. Understanding how to draw and interpret the Lewis structure is crucial for mastering concepts in chemistry, such as molecular geometry, bonding, and polarity.
By following the steps outlined above, you can easily draw the Lewis structure for phosphorus trichloride and understand its molecular properties. Whether you’re a student or a chemistry enthusiast, mastering the PCl₃ Lewis structure will give you deeper insight into the behavior of molecules and their interactions.
One Comment