Understanding the ICL4- Lewis Structure
Introduction to the ICl4− Ion
The ICl4−ion consists of one iodine (I) atom and four chlorine (Cl) atoms, with an overall negative charge. Understanding the Lewis structure for this polyatomic ion can help us visualize its bonding, geometry, and the placement of electrons. In this ion, iodine is the central atom surrounded by chlorine atoms, and the entire molecule carries a negative charge.
Draw CO2 Lewis Structure to get more skills and pass the test with good grades
Step-by-Step Guide to Drawing the Lewis Structure for ICl4−
Let’s go through the process of drawing the Lewis structure for ICl4− in a clear, step-by-step way.
Step 1: Count the Total Valence Electrons
- Iodine (I) has 7 valence electrons because it belongs to Group 17 in the periodic table.
- Each Chlorine (Cl) atom also has 7 valence electrons, and there are 4 chlorine atoms in ICl4−
So, the total number of valence electrons from iodine and chlorine atoms is calculated as follows:7 (from I)+4×7 (from each Cl)=7+28=35 valence electrons7 \, + 4 \times 7 \, = 7 + 28 = 35 \, \text{valence electrons}7(from I)+4×7(from each Cl)=7+28=35valence electrons
- Account for the Negative Charge (-1): The negative charge means we add one extra electron.
This brings the total to:35+1=36 valence electrons35 + 1 = 36 \, \text{valence electrons}35+1=36valence electrons
Step 2: Arrange the Atoms
- Iodine will be the central atom because it can expand its octet (it’s in Period 5) and can hold more than 8 electrons.
- Place the four chlorine atoms around iodine, one on each side.
Step 3: Form Bonds Between Iodine and Chlorine Atoms
- Draw single bonds between the iodine atom and each of the four chlorine atoms. Each single bond represents a pair of shared electrons (2 electrons per bond).
After forming these four single bonds, we use 4×2=84 \times 2 = 84×2=8 electrons out of our 36 total valence electrons:36−8=28 electrons remaining36 – 8 = 28 \, \text{electrons remaining}36−8=28electrons remaining
Step 4: Distribute the Remaining Electrons to Complete Octets for Chlorine Atoms
- Place the remaining electrons around each chlorine atom to complete their octets. Each chlorine needs 8 electrons in total (2 from the bond and 6 as lone pairs).
Since each chlorine already has 2 bonding electrons, each needs 6 additional electrons (3 lone pairs) to complete its octet.
- Distribute 6 electrons to each chlorine atom, totaling 4×6=244 \times 6 = 244×6=24 electrons.
After completing the octets for chlorine, we have:28−24=4 electrons remaining28 – 24 = 4 \, \text{electrons remaining}28−24=4electrons remaining
Step 5: Place Remaining Electrons on the Central Iodine Atom
- Place the remaining 4 electrons on the central iodine atom as two lone pairs.
This completes the electron distribution, with all valence electrons accounted for.
Step 6: Check the Octet Rule and Formal Charges
- Octets: Each chlorine atom has 8 electrons (2 bonding + 6 lone-pair electrons).
- Iodine has 12 electrons around it (8 from bonds with chlorine and 4 from lone pairs). Since iodine can expand its octet, this configuration is allowed.
- Formal Charge Calculation: Checking the formal charge can verify that this structure is stable.
- For each chlorine: 7 (valence electrons)−6 (nonbonding)−1 (bond)=07 \, (\text{valence electrons}) – 6 \, (\text{nonbonding}) – 1 \, (\text{bond}) = 07(valence electrons)−6(nonbonding)−1(bond)=0.
- For iodine: 7−4 (nonbonding)−4 (bonds)=−17 – 4 \, (\text{nonbonding}) – 4 \, (\text{bonds}) = -17−4(nonbonding)−4(bonds)=−1.
The negative formal charge is on the central iodine atom, matching the overall charge of −1-1−1 for the ion.
Final Lewis Structure for ICl4−
The completed Lewis structure for ICl4−\text{ICl}_4^-ICl4− can be represented as follows
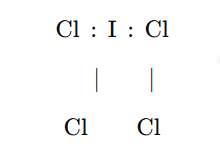
In this structure:
- Iodine (I) is in the center, bonded to each of the four chlorine (Cl) atoms.
- Each Cl atom has three lone pairs of electrons around it to satisfy its octet.
- The iodine atom has two lone pairs of electrons on it, which, along with the bonds to chlorine, gives iodine a total of 12 electrons. This expanded octet is possible because iodine is in the third period or higher in the periodic table.
The complete structure reflects all 36 electrons and the single negative charge.
Molecular Geometry and Shape of ICl4−\text{ICl}_4^-ICl4−
The geometry of ICl4−\text{ICl}_4^-ICl4− is influenced by the lone pairs on the central iodine atom. To determine the shape:
- Electron Pair Arrangement: There are four bonding pairs (each bond with chlorine) and two lone pairs around the central iodine atom. According to VSEPR (Valence Shell Electron Pair Repulsion) theory, this arrangement—six regions of electron density—results in an octahedral electron geometry.
- Molecular Shape: Due to the two lone pairs on iodine, the actual shape of ICl4−\text{ICl}_4^-ICl4− is square planar. The lone pairs are positioned opposite each other, while the four chlorine atoms form a square around the iodine in a flat, planar arrangement.
- Bond Angles: In a square planar geometry, the bond angles between adjacent chlorine atoms are 90°.
Polarity of ICl4−\text{ICl}_4^-ICl4−**
The ICl4−\text{ICl}_4^-ICl4− ion is nonpolar overall, even though each I–Cl bond is polar. In the square planar arrangement, the dipoles from the four I–Cl bonds cancel each other out because they are symmetrically arranged around the iodine atom. This symmetry results in a nonpolar molecule despite individual polar bonds.
Summary of Key Points
- Total Valence Electrons: 36 (7 from iodine, 7 from each of the four chlorines, plus one additional electron for the negative charge).
- Lewis Structure: Iodine in the center with four chlorine atoms attached by single bonds, and two lone pairs on iodine.
- Molecular Geometry: Square planar, with an octahedral electron pair geometry.
- Polarity: Nonpolar due to the symmetric square planar shape.
This structure and geometry help explain the properties of the ICl4−\text{ICl}_4^-ICl4− ion, including its nonpolarity and stability in certain chemical environments. Understanding these factors is crucial for predicting its behavior in chemical reactions and interactions.